The research of the Saunders lab is focused on the study of cell division and the origin of divisional defects in cancer cells. When cells divide, it is essential that the components of the cell are separated such that both daughters will inherit the necessary starting material to propagate as healthy cells. During tumorigenesis, cells often divide irregularly with missegregation of chromosomes and microtubule organizing centers or centrosomes. Both of these changes are thought to contribute to the enhanced mutational rates contributing to tumor progression. Our interests are to define pathways required for normal division and determine how these pathways are altered in human disease.
Currently, we are focused on three types of abnormal division in tumor cells. These are multipolar spindles, failed cytokinesis and anaphase bridges.
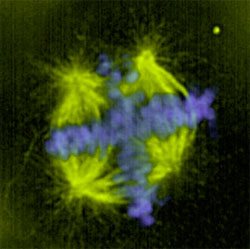
Multipolar spindles happen when the cell tries to divide the chromosomes into more than two groups. This is a result of amplification and unclustering of the centrosomes in the cell. Centrosomes organize the microtubules into a spindle to separate the chromosomes during mitosis. Extra centrosomes can form more than two microtubule foci resulting in abnormal separation of the chromosomes into three or more groups (Figure 1). In cancer cells, multipolarity is achieved by interference with the microtubule motor called cytoplasmic dynein. Dynein is not visible on cancer cell spindles and inhibition of dynein can greatly increase the multipolarity resulting from centrosomal amplification. In some tumor cells, dynein is inhibited by overexpression of another spindle motor called NuMA.
A related question is how the centrosomes get amplified in the first place. Recent work in the lab has shown that centrosomes are amplified by failure of cytokinesis, the divisional process that occurs to the cell at the end of mitosis. Cytokinesis was shown to fail in cancer cells due to underphosphorylation of the regulatory myosin light chain. We believe this inhibition occurs in cancer cells by overexpression of the cell cycle regulatory kinase Aurora B that phosphorylates the myosin light chain kinase reducing its abundance, localization and biochemical activity. Deficient myosin light chain phosphorylation causes the cleavage furrow to regress.
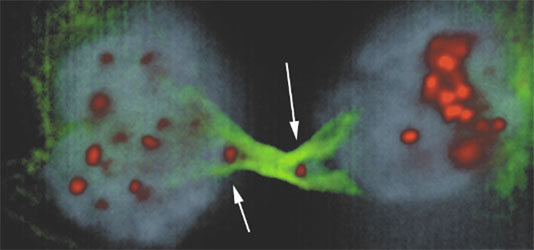
The third divisional defects of cancer cells we study is anaphase bridges. These structures are a result of dicentric chromosomes attaching to both centrosomes and result in a tug-of-war between two dividing cells for a single chromosome (Figure 2). Our current focus is in role of chromatin bridges in activating Aurora B to inhibit myosin light chain kinase leading to failure of cytokinesis and centrosomal amplification.
Sankunny M, Parikh RA, Lewis DW, Gooding WE, Sa
Sankunny M, Parikh RA, Lewis DW, Gooding WE, Saunders WS, Gollin SM. (2014) Targeted inhibition of ATR or CHEK1 reverses radioresistance in oral squamous cell carcinoma cells with distal chromosome arm 11q loss. Genes Chromosomes Cancer 53(2):129-43
Bartoli KM, Bishop DL, Saunders WS. (2011) The
Bartoli KM, Bishop DL, Saunders WS. (2011) The role of molecular microtubule motors and the microtubule cytoskeleton in stress granule dynamics. Int J Cell Biol. v.2011:939848
Wu, Q., F.L. Xu, Y. Li, E.V. Prochownik, and W.S. Saunders (2010) The c-Myc target glycoprotein1b
Wu, Q., F.L. Xu, Y. Li, E.V. Prochownik, and W.S. Saunders (2010) The c-Myc target glycoprotein1balpha links cytokinesis failure to oncogenic signal transduction pathways in cultured human cells. PLoS One 5:e10e10819
Wu, Q., R.M. Sahasrabudhe, L.Z. Luo, D.W. Lewis, S.M. Gollin, and W.S. Saunders (2010) Deficiency
Wu, Q., R.M. Sahasrabudhe, L.Z. Luo, D.W. Lewis, S.M. Gollin, and W.S. Saunders (2010) Deficiency in myosin light-chain phosphorylation causes cytokinesis failure and multipolarity in cancer cells. Oncogene 29:4183-4193
Xu, F.L., Y. Rbaibi, K. Kiselyov, J.S. Lazo, P. Wipf, and W.S. Saunders (2010) Mitotic slippage i
Xu, F.L., Y. Rbaibi, K. Kiselyov, J.S. Lazo, P. Wipf, and W.S. Saunders (2010) Mitotic slippage in non-cancer cells induced by a microtubule disruptor, disorazole C1. BMC Chem Biol 10:11
Li, Y., F.L. Xu, J. Lu, W.S. Saunders, and E.V. Prochownik (2010) Widespread genomic instability
Li, Y., F.L. Xu, J. Lu, W.S. Saunders, and E.V. Prochownik (2010) Widespread genomic instability mediated by a pathway involving glycoprotein Ib alpha and Aurora B kinase. J Biol Chem 285:13183-13192
Tierno, M.B., C.A. Kitchens, B. Petrik, T.H. Graham, P. Wipf, F.L. Xu, W.S. Saunders, B.S. Raccor
Tierno, M.B., C.A. Kitchens, B. Petrik, T.H. Graham, P. Wipf, F.L. Xu, W.S. Saunders, B.S. Raccor, R. Balachandran, B.W. Day, J.R. Stout, C.E. Walczak, A.P. Ducruet, C.E. Reese, and J.S. Lazo (2009) Microtubule binding and disruption and induction of premature senescence by disorazole C(1). J Pharmacol Exp Ther 328:715-722
Acilan, C., and W.S. Saunders (2008) A tale of too many centrosomes. Cell 134
Acilan, C., and W.S. Saunders (2008) A tale of too many centrosomes. Cell 134:572-575
Xu, F.L., and W.S. Saunders (2008) Actin and microtubules: working together to control spindle po
Xu, F.L., and W.S. Saunders (2008) Actin and microtubules: working together to control spindle polarity. Cancer Cell 14:197-199
Parikh, R.A., J.S. White, X. Huang, D.W. Schoppy, B.E. Baysal, R. Baskaran, C.J. Bakkenist, W.S.
Parikh, R.A., J.S. White, X. Huang, D.W. Schoppy, B.E. Baysal, R. Baskaran, C.J. Bakkenist, W.S. Saunders, L.C. Hsu, M. Romkes, and S.M. Gollin (2007) Loss of distal 11q is associated with DNA repair deficiency and reduced sensitivity to ionizing radiation in head and neck squamous cell carcinoma. Gene Chromosome Canc. 46:761-775
Acilan, C., D.M. Potter, and W.S. Saunders (2007) DNA repair pathways involved in anaphase bridge
Acilan, C., D.M. Potter, and W.S. Saunders (2007) DNA repair pathways involved in anaphase bridge formation. Gene Chromosome Canc. 46:522-531
Reshmi, S.C., S. Roychoudhury, Z. Yu, E. Feingold, D. Potter, W.S. Saunders, and S.M. Gollin (200
Reshmi, S.C., S. Roychoudhury, Z. Yu, E. Feingold, D. Potter, W.S. Saunders, and S.M. Gollin (2007) Inverted duplication pattern in anaphase bridges confirms the breakage-fusion-bridge (BFB) cycle model for 11q13 amplification. Cytogenet. Genome Res. 116:46-52
Reshmi, S.C., X. Huang, D.W. Schoppy, R.C. Black, W.S. Saunders, D.I. Smith, and S.M. Gollin (200
Reshmi, S.C., X. Huang, D.W. Schoppy, R.C. Black, W.S. Saunders, D.I. Smith, and S.M. Gollin (2007) Relationship between FRA11F and 11q13 gene amplification in oral cancer. Gene Chromosome Canc. 46:143-154
Mondal, G., S. Sengupta, C.K. Panda, C.K. Gollin, W.S. Saunders, and S. Roychoudhury (2007) Overe
Mondal, G., S. Sengupta, C.K. Panda, C.K. Gollin, W.S. Saunders, and S. Roychoudhury (2007) Overexpression of Cdc20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis 28:81-92
Saunders, W. (2005) Centrosomal amplification and spindle multipolarity in cancer cells. Semi
Saunders, W. (2005) Centrosomal amplification and spindle multipolarity in cancer cells. Semin. Cancer Biol. 15:25-32
Sproul, L.R., D.J. Anderson, A.T. Mackey, W.S. Saunders, and S.P. Gilbert (2005) Cik1 targets the
Sproul, L.R., D.J. Anderson, A.T. Mackey, W.S. Saunders, and S.P. Gilbert (2005) Cik1 targets the minus-end kinesin depolymerase Kar3 to microtubule plus ends. Curr. Biol. 15:1420-1427
Quintyne, N.J., J.E. Reing, D.R. Hoffelder, S.M. Gollin, and W.S. Saunders (2005) Spindle multipo
Quintyne, N.J., J.E. Reing, D.R. Hoffelder, S.M. Gollin, and W.S. Saunders (2005) Spindle multipolarity is prevented by centrosomal clustering. Science 307:127-129
Dr. Saunders received his Ph.D. in 1990 from the Johns Hopkins University, performed his postdoctoral studies with M.A. Hoyt at Johns Hopkins University, and joined the Department in 1994.



